Sepsis is one of the most significant causes of premature death in the world. In the UK there are 120,000 critical care unit admissions and 44,000 deaths per annum attributed to sepsis. 14,000 of these deaths are thought to be preventable through improved diagnosis and reduced treatment delays.
The Laser Spectroscopy Group at RAL Space have developed an instrument, the Laser Isotope Ratiometer (LIR), that builds on RAL Space's expertise in innovative space technologies to provide a more efficient diagnosis tool for sepsis in hospital patients using a simple breath test.
The LIR was developed to study gases in the Martian atmosphere. Its compact, lightweight and robust design that makes it ideal for use on a spacecraft, has also meant that it could be adapted into a portable medical device. Because it can be used at the bedside, it is easier for nurses to use and administer compared to existing laboratory-scale equipment currently used to diagnose sepsis.
The device will be able to provide results instantly, helping doctors start treatment earlier which could reduce the number of sepsis linked deaths. The breath test is non-invasive, making it safer for frequent use by at risk patients than blood tests which require an extended waiting period due to laboratory analysis.
On entry to hospital, patients breathe into a bag where the LIR uses beams of laser light to interrogate the gas samples. The instrument measures the concentration of two isotopes of carbon found in the sample: carbon-13 and carbon-12, which are exhaled respectively as the isotopologues 13CO2 and 12CO2.
"By measuring a change over time in the concentrations of exhaled 13CO2 and 12CO2 relative to each other, metabolic changes can be inferred. Under controlled conditions, these changes were shown to be indicative of sepsis" said Dr Damien Weidmann, Head of the Laser Spectroscopy Group at RAL Space.
"The onset of sepsis can change the ratio of 13CO2 and 12CO2 in the breath because of how the body's immune system responds to infection through the acute phase response. This response involves a series of processes that prefer to use carbon-13 instead of carbon-12, causing the exhaled amount of 13CO2 and 12CO2 in the breath to differ from its healthy levels".
Dr Weidmann explains: “If the beam of laser light is adjusted to a certain frequency, the carbon dioxide molecules will absorb energy from it and become excited. This stops the light from transmitting through fully. The detectors in the instrument then measure the intensity of laser light that does transmit through the sample. The more 13CO2 or 12CO2 molecules there are in the line of the laser beam, the less light will be transmitted through".
The initial concept of using carbon isotope measurements in exhaled breath as a way to detect sepsis was discovered by scientists at the University of Wisconsin-Madison. Dr Weidmann's team applied these findings to their own device, working with researchers led by Dr Mark McPhail at King's College London to the stage where the LIR is now undergoing pilot clinical studies at King's College Hospital in London.
"Working with RAL Space has been a fantastic opportunity to use their expertise to benefit some of the sickest patients in hospitals" said Dr Mark McPhail, Senior Lecturer & Consultant in Liver Critical Care & Hepatology, Kings College London & Kings College Hospital.
"When patients have difficult to treat infections and need to come to intensive care units, they have a high risk of death. Earlier detection can save lives and a breath test that can be performed simply and cheaply at the bedside could greatly improve our ability to detect sepsis".
Measuring minute changes in isotopic ratios has proven to be challenging. However, features developed at RAL Space such as an optical switch, which ensures that 'background noise' or temperature deviations do not affect the measurements and that the instrument is continuously calibrated to its environment, make the LIR highly reliable. Following successful clinical trials, the LIR could transform medical diagnosis of sepsis throughout the UK through the provision of accurate and real-time measurements.
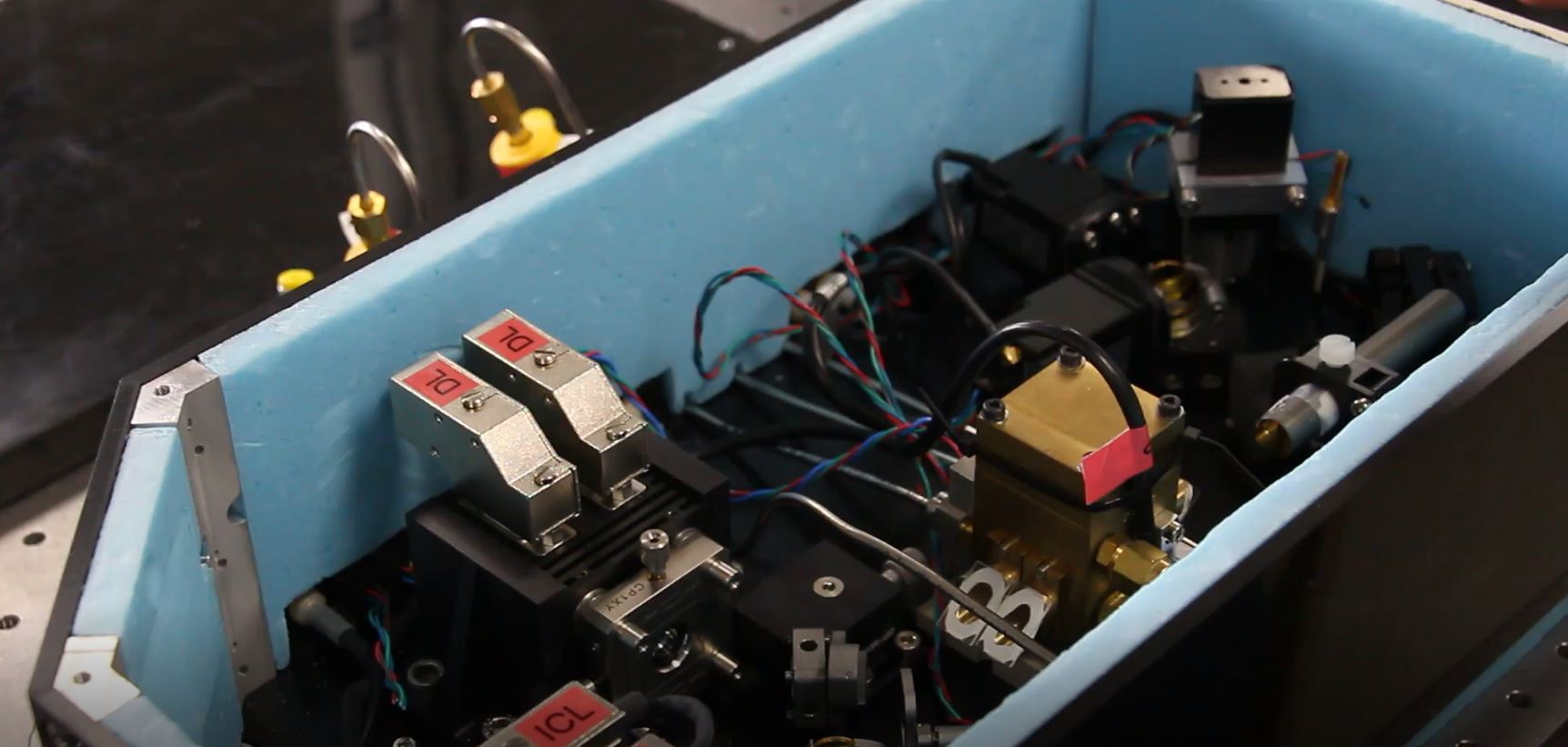 The Laser Isotope Ratiometer in RAL Space's Laser Spectroscopy Facility.
The Laser Isotope Ratiometer in RAL Space's Laser Spectroscopy Facility.
The science behind the laser isotope ratiometer:
The LIR works out the proportions of carbon dioxide isotopologues within gas samples. It detects the amount of 13CO2:12CO2 in a patient's breath by passing a laser beam through the gas sample. At the same time, a split part of the laser also passes through a reference gas sample where the isotopic ratio is already known. The laser beams probe each sample by performing measurements over a small range of wavelengths in the infrared spectrum called the fingerprint region (2-20µm). The detectors at the end of the instrument measures the intensity of laser light transmitted through the sample, and creates a graph that is used as a 'fingerprint' to be compared to the reference sample.
Isotope: atoms of the same element which contain the same number of protons but different number of neutrons.
Isotopologue: a molecule in which one or more of the atoms in the molecule is replaced by its isotope. 13CO2 and 12CO2 are isotopologues of carbon dioxide and differ only in the isotope of carbon that makes up the molecule. Owing to the high precision of the laser light which probes the isotopologues, the LIR can distinguish between 13CO2 and 12CO2 as it can be tuned to excite one but not the other, as 13CO2 or 12CO2 have slight mass differences so have different frequencies that they vibrate at.
Fingerprint region: This region of the infrared spectrum is where most molecules have energy states that cause them to vibrate and rotate. By exposing molecules to laser light in this region, they will absorb energy and temporarily store energy.
Fingerprint: From the 'fingerprint' graph, the proportions of the two isotopologues in the gas sample is worked out by measuring the absorption on a 13CO2 line and a 12CO2 line. The more isotopologues of CO2 there are in the line of sight of the laser beam, the stronger the absorption of light there will be because more photons will be taken out to excite the molecules. The strength of absorption of the transmitted laser light is therefore proportional to isotopologue concentration.
Stock image of blood close up. Image credit: Pixabay
Bringing space down to Earth: The work done at RAL Space isn't just restricted to exploring and understanding the space that surrounds our planet, it's also about feeding space science, technology and data back down to Earth. Our staff are involved in a number of exciting projects that have used their roots in space and satellite technology to develop products and services with important applications for life on Earth.